Is your clinic discarding viable embryos? The uncomfortable truth
Is your clinic discarding viable embryos? The uncomfortable truth. Learn why 1PN, 2.1PN, 3PN and segmental aneuploid embryos have potential and need clinical consideration.
An embryologist's duty is not to find the perfect embryo, it's to do everything in their power to uncover the true potential of every embryo.
One thing I have learned in almost 24 years of working as an embryologist is this:
Embryos don't know the rules we have created for them. They haven’t read the textbooks. They don’t neatly follow our grading systems. They can do unexpected weird things in the laboratory and still go on to do exactly what they are meant to do — create a healthy human being.
The time has come to say this out loud: Viable embryos are still being discarded simply because some embryology teams are not keeping pace with developments in the science. In some cases, processes simply haven’t evolved. In others, outdated equipment is being used, equipment that doesn’t allow us to see what we now know we need to see to discover the true facts.
This post is intended to be helpful, not scary. Its purpose is to support you through an already overwhelming and emotionally challenging journey, and to help you recognise when something you are being told may not be factually correct or scientifically current.
From segmental aneuploidy, to abnormally (or atypically) fertilised embryos, to the real and measurable benefits of time-lapse technology, our understanding in the last couple of years has changed dramatically.
Let me explain what we know now that we didn’t know before, and where the science genuinely stands today.
Abnormally fertilised embryos
Embryos are meant to show us 2 nucleus’s (known as PN’s) between the hours of 12-22 hours after they have been created by ICSI or IVF. Those nucleus’ then merge and disappear in a process called syngamy.
It's at this point they go back to looking like a non fertilised egg. The process of syngamy normally happens around 22-24 hours after creation of the embryo.
But what about when they don't do this in the time they are meant to or in the way the textbooks tells us? Is it really that simple, are they really non-viable? We now call them atypical instead of abnormal ... or at least we should.
Atypically fertilised embryos
The normal 2PN
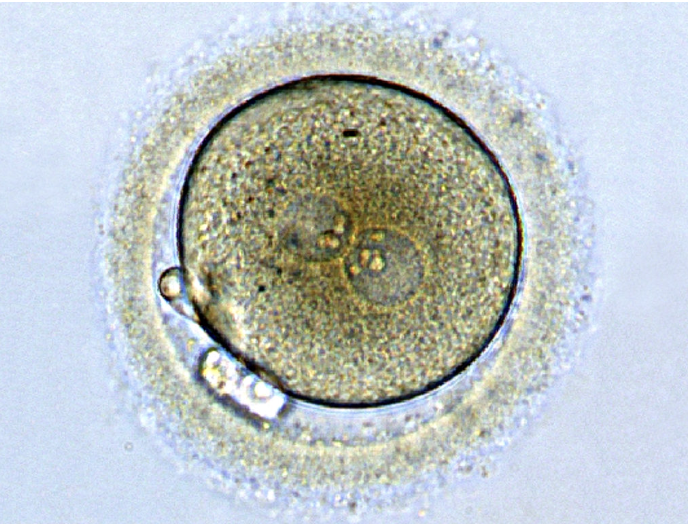
The normal 2PN is a normally fertilised embryo. This is what the textbooks tell us we should see and this is what we do see 90% of the time when correct fertilisation has taken place.
What about the other 10%?
The 1PN
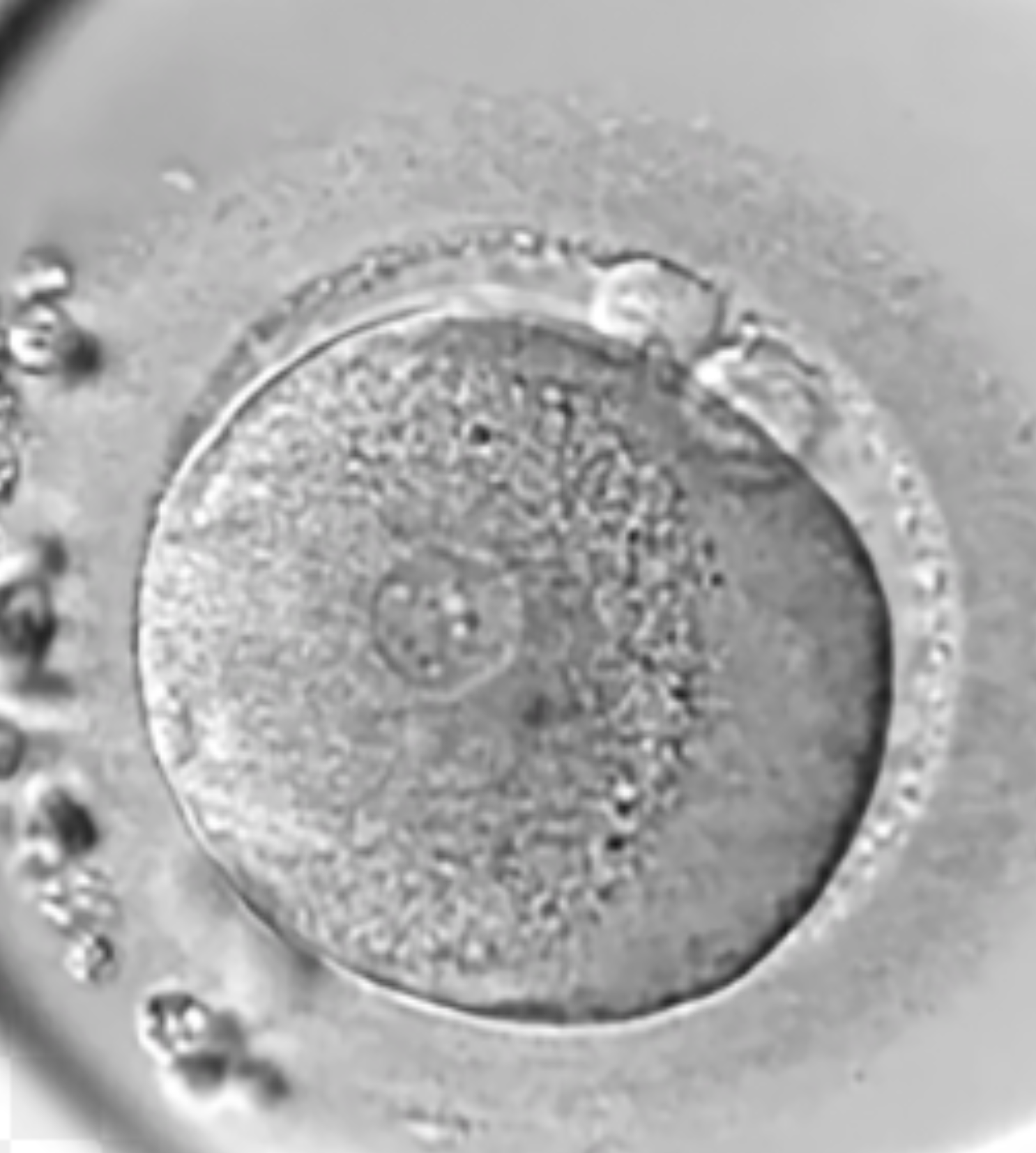
The 1PN embryos have good potential for being normal. Around 50-60% of embryos that show a 1PN after IVF are normally fertilised. These should not simply be discarded without discussion. They have potential.
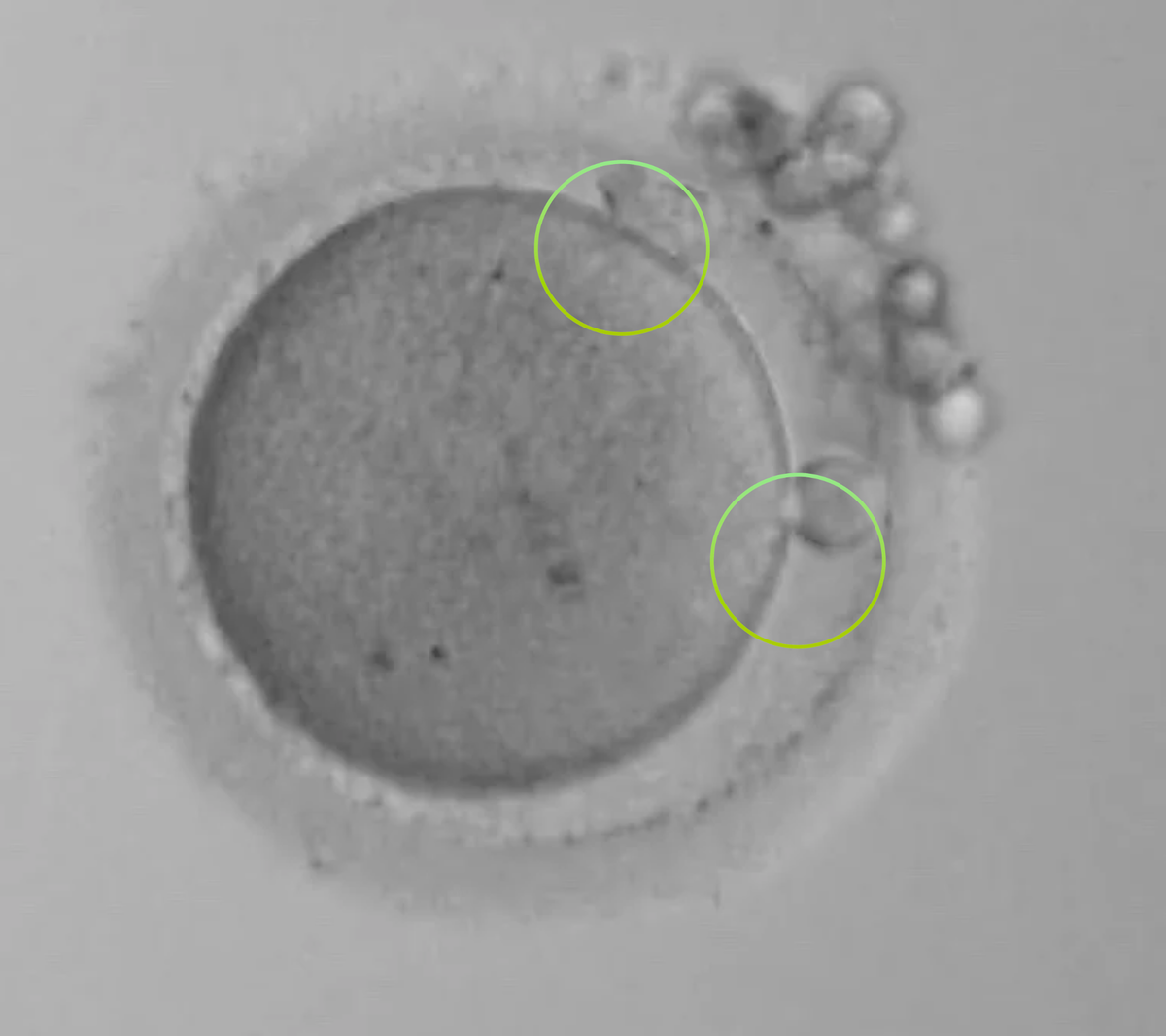
The 0PN
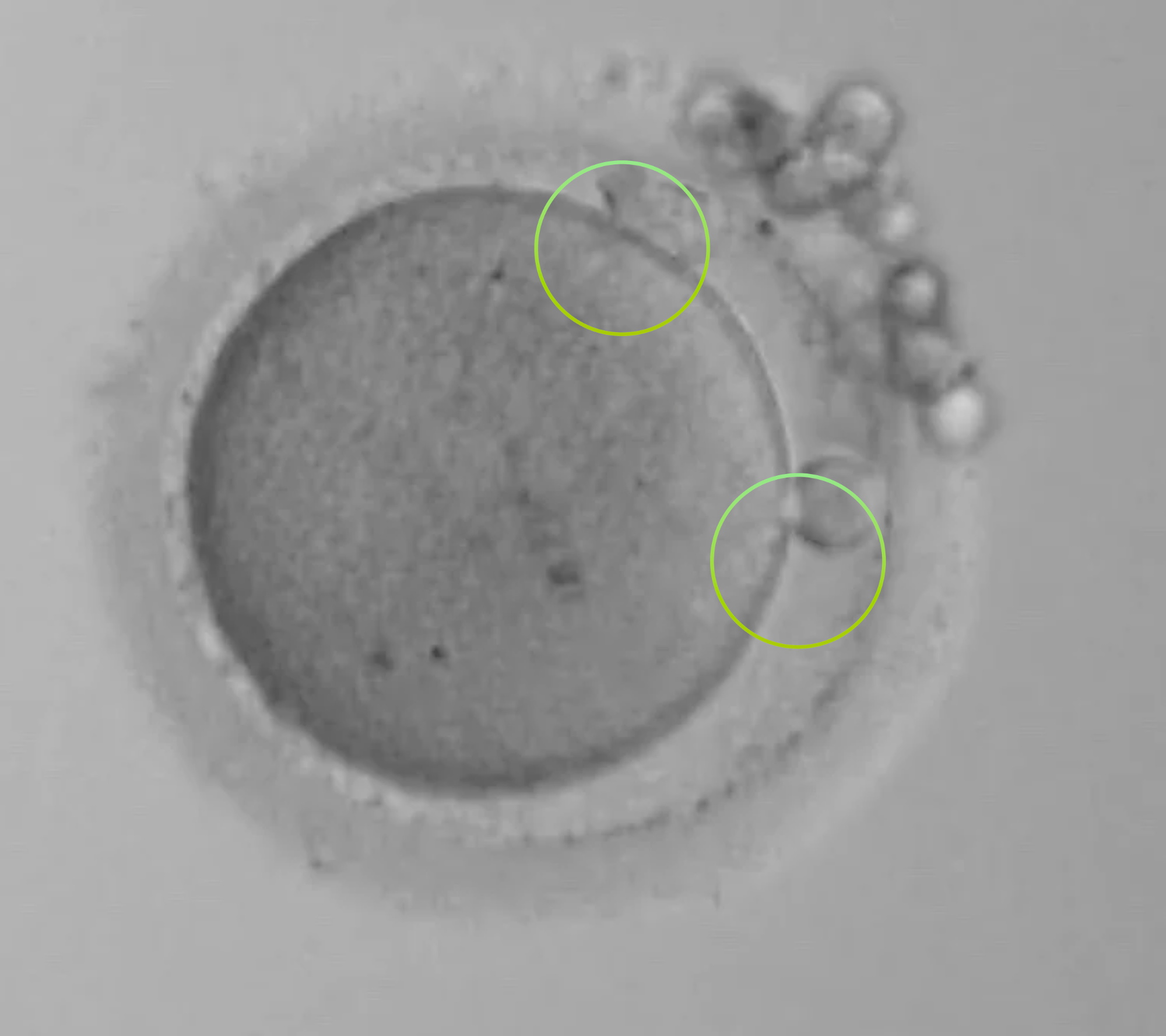
With a 0PN you cannot see any PN’s but you can see 2 Polar bodies (the circles on the outside). In a closed (non-timelapse) incubator system these have to be kept to check for development, as you might have just ‘missed’ the visuals ... did syngamy happen early?
The 3PN
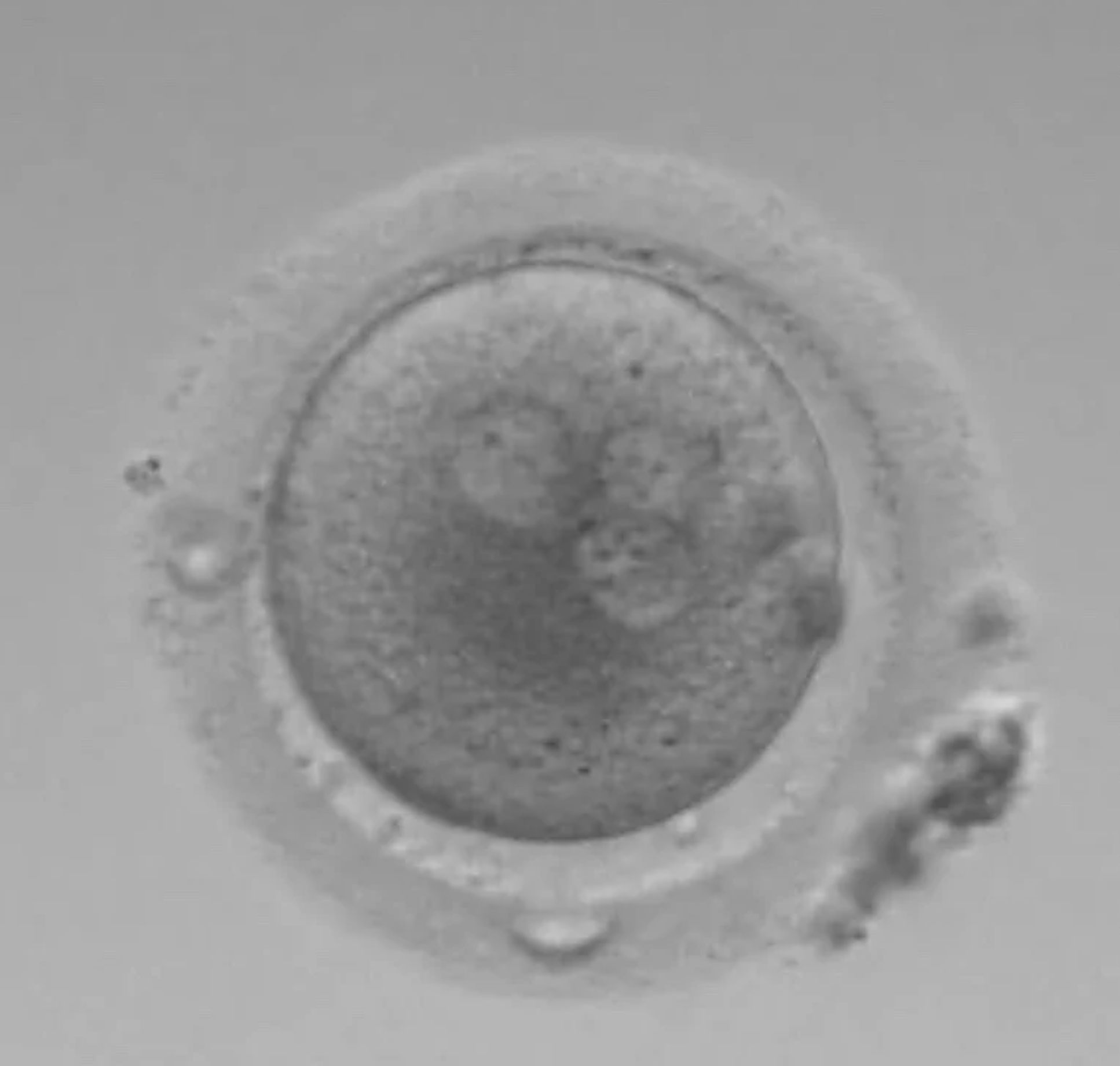
This is a clear 3PN. The PN’s are all the same size. These embryos are impossible to use without PGT-A testing to confirm how much DNA they have in them. True 3PN’s (with 3 copies of DNA) can result in dangerous partial molar pregnancies.
The 2.1PN
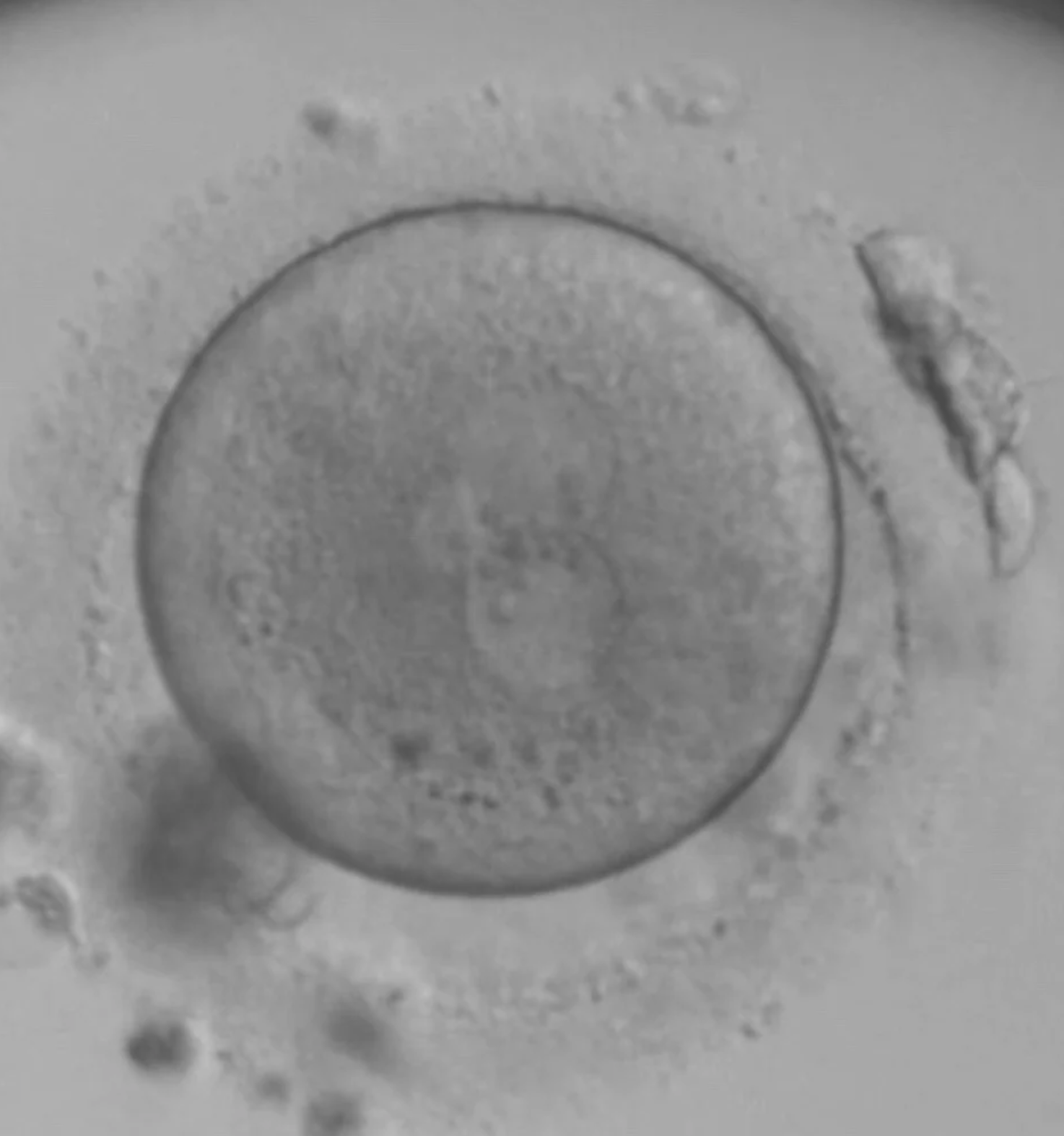
You might also think this is a 3PN ... it is not. The size of the PN’s are different with one significantly smaller. These are normally fertilised 90% of the time. But they need PGT-A to be sure that they can be used safely.
Segmental aneuploid embryos
We know whole chromosome aneuploid embryos, tested on SNP based PGT-A technologies, like JUNO, do not result in live births* (Tiegs et al 2021. New England Journal of Medicine).
But this truth is not the same for segmental aneuploid embryos where only a part of the chromosome is reported as lost or gained embryo.
20-25% of segmental aneuploid embryos can result in live births, and although they are not without challenges and concerns, we cannot simply just discard and discount them without deeper conversation.
They might be the ONLY embryo available to us.
Timelapse and what it tells us
Timelapse incubators are much more than just incubators and are one of the most important technologies we have. They allow us to see everything. A lot of the speculation around atypical fertilisation can be understood clearly from watching the video of an embryo developing.
The potential for an embryo to be non-viable can sometimes be identified as early as the first cleavage division, but only if you get to watch it happen. This is especially important in clinics doing Day 3 transfers.
Please can someone explain to me why this magnificent tool is considered an add on and not a necessity that every patient should have access to?
In summary
There are no longer any valid excuses for not exploring what was once considered impossible to explore. PGT-A allows us to rescue atypically fertilised embryos from unnecessary discard. Timelapse technology allows us to observe a wide range of developmental events we simply didn’t know existed.
To my fellow embryologists: our duty is not to chase the perfect textbook embryo. Our responsibility is to use every tool available to understand the full potential of every embryo. By doing so, we minimise the risk of discarding something with even limited potential, because that one unexplainable embryo may represent a patient’s only chance.
Confused by all the terminology? View the IVF glossary of terms.
